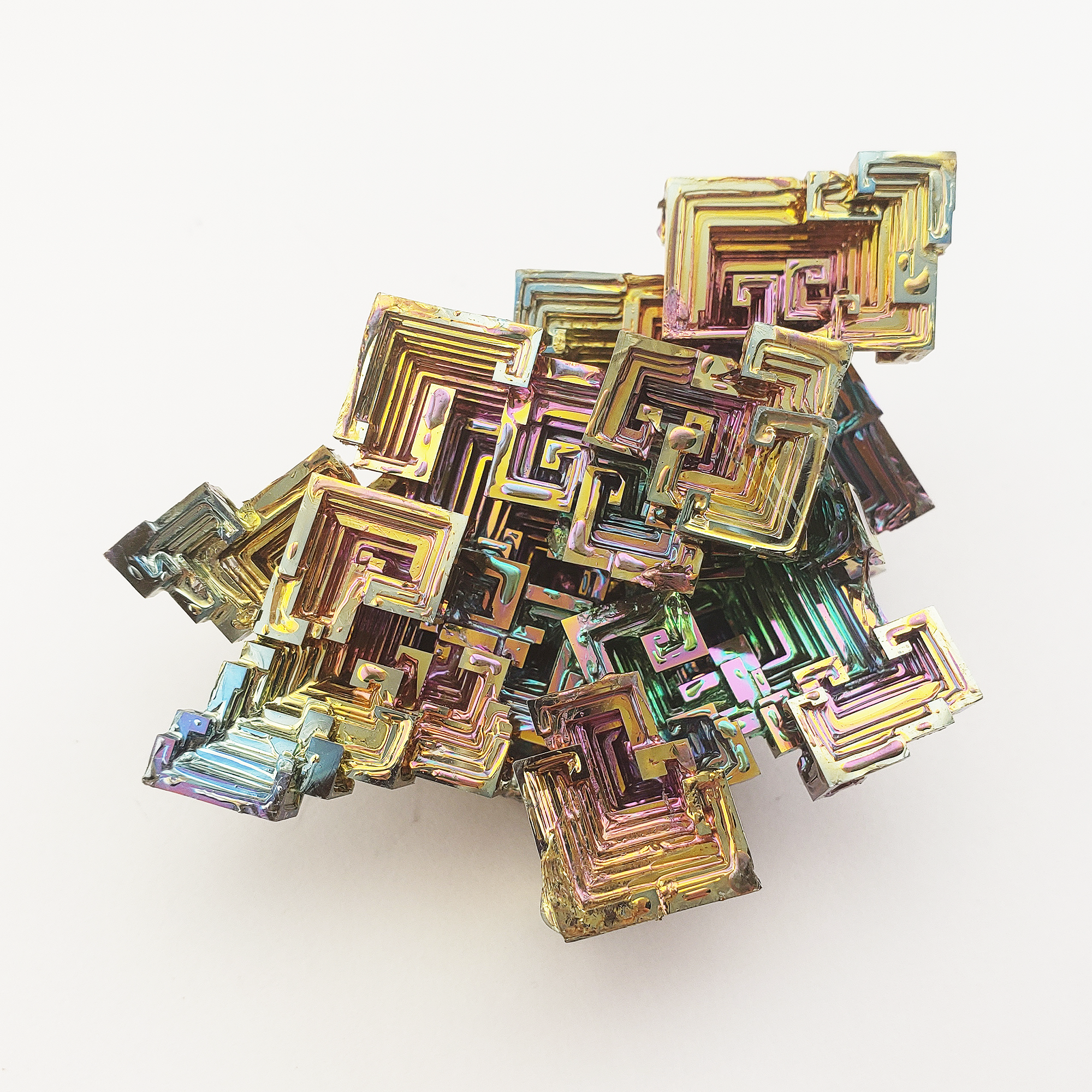
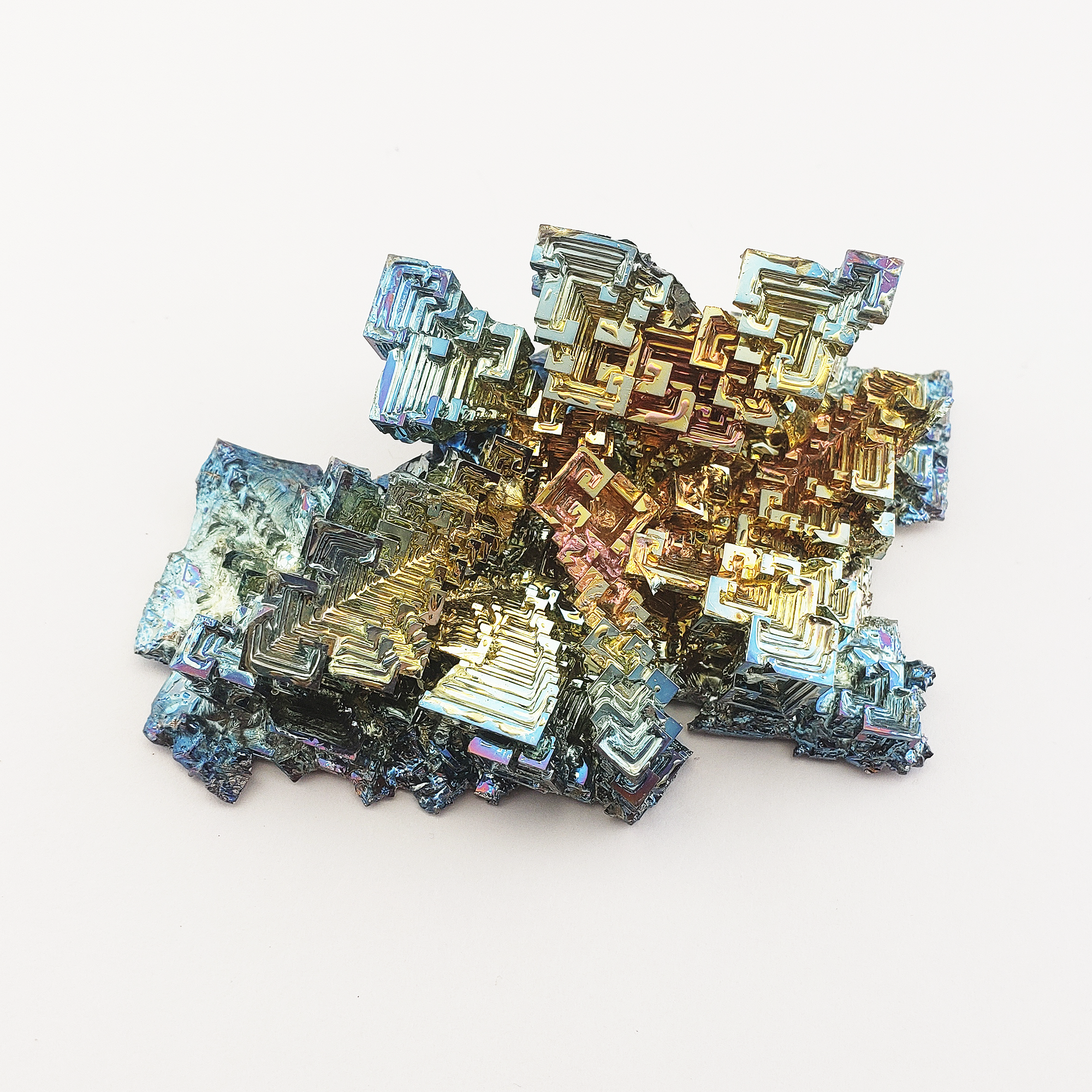
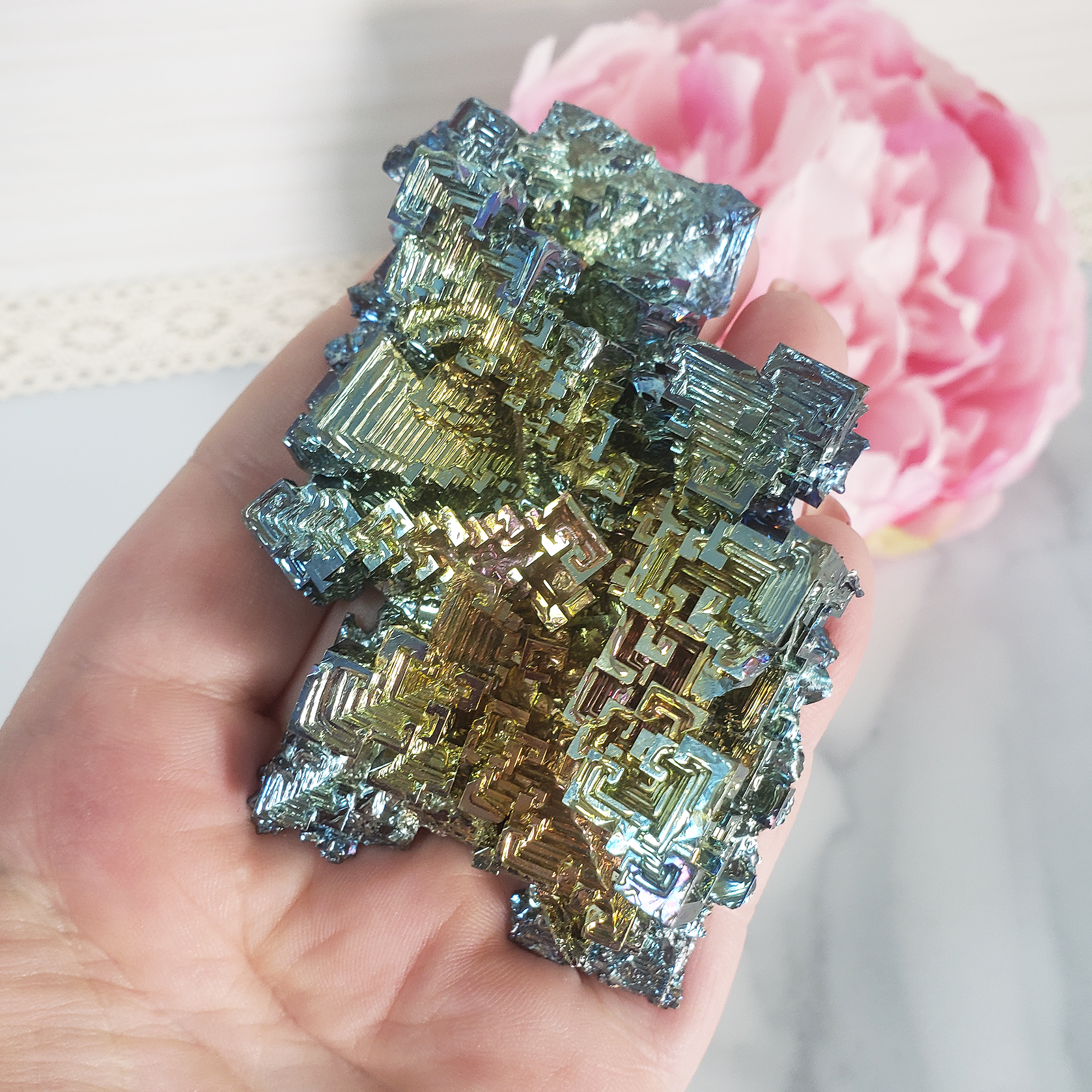
What is Bismuth?
In the terms of crystal collectors, when we talk about Bismuth crystals, we are specifically referring to high purity Bismuth clusters which are grown in labs. This is because naturally occurring Bismuth does not have the same rainbow crystal luster that crystal collectors typically associate with the word Bismuth. As a natural element, Bismuth is usually white to reddish, and since it is typically included by additional materials it doesn't form the highly geometric maze patterns found in rainbow Bismuth crystals, although it can still tarnish to faint shades of yellow, pink, or blue.
Is Bismuth a metal? Yes! Although it is often referred to as a crystal, Bismuth is considered a brittle metal, with a high Mohs hardness.
On the Mohs scale, it is rated between 2.0 and 2.5, indicating a relatively soft mineral compared to others.
In addition to the Mohs scale, bismuth's hardness is also measured using the Vickers hardness test, where it scores between VHN100=16 and 18 kg/mm². This further emphasizes its brittle nature, providing a comprehensive understanding of its mechanical properties.
Understanding these values helps in various applications, particularly when considering how bismuth interacts with other materials in industrial processes.
This means it must be handled carefully to prevent damage, since a knock against a hard surface can cause Bismuth to break. At standard pressure and temperature, Bismuth presents itself as a solid, silvery-grey metal.
While its appearance suggests strength, it's important to note that Bismuth is also known for its unique combination of being difficult to cut yet easily broken. This duality makes it an intriguing material in various applications. Despite its strength, a simple hammer strike can easily fracture the metal, highlighting its fragile nature.
Understanding these characteristics is crucial for industries that utilize Bismuth, ensuring safe and effective handling.
What is the Origin of the Name of Bismuth?
The name "Bismuth" has its roots in the German language. It is derived from the term "Weisse masse," which translates to "white mass," highlighting the element's original color. Over time, "Weisse masse" evolved into "Wasmuth," which eventually led to the modern name "Bismuth." This progression reflects the historical linguistic shifts that influenced its current nomenclature.
Purity Levels and Forms of Bismuth Metal Samples
Bismuth metal is renowned for its high purity, often reaching 99.99%, making it an ideal element for collectors and scientific applications alike. Here’s a closer look at the various forms and their uses:
-
Shiny and Colorful Pieces: Available in weights like 5 grams or 10 grams, these high-purity samples are perfect for element collections. They come in glass vials with labels, ensuring a pristine presentation for collectors.
-
Density Cubes: Standard cubes measuring 10x10x10mm, made with 99.99% pure Bismuth, weigh approximately 9.78 grams. These are fantastic for educational settings, such as science classrooms and universities, providing a tangible way to explore the properties of Bismuth.
-
Crystals: High purity Bismuth crystals exhibit an amazing colored surface and are available in various weights. Single crystal forms are offered in glass vials, with weights ranging from 2 to 12 grams, providing a vibrant display for any collection.
-
Crystalline Pieces: Packed into vials weighing up to 35 grams, these pieces offer a dazzling array of geometric beauty, highlighting the unique characteristics of lab-grown Bismuth.
-
Rods and Ingots: Available as shiny rods or ingots for both collection and laboratory use. These forms also allow for the melting and creation of custom Bismuth crystals, expanding their utility.
-
Large Display Cubes: For those looking for a bold centerpiece, 1 inch cubes of 99.99% pure Bismuth offer substantial weight and a striking presence, perfect for display in educational or professional settings.
This diversity in forms and consistent purity level ensures that Bismuth metal samples cater to a wide array of interests, from passionate hobbyists to professional educators.
How are Bismuth Rainbow Crystals Formed?
In terms of crystal collectors, when we talk about Bismuth crystals, we are specifically referring to high-purity Bismuth clusters which are grown in labs. This is because naturally occurring Bismuth does not have the same rainbow crystal luster that crystal collectors typically associate with the word Bismuth. As a natural element, Bismuth is usually white to reddish, and since it is typically included by additional materials, it doesn't form the highly geometric maze patterns found in rainbow Bismuth crystals, although it can still tarnish to faint shades of yellow, pink, or blue.
To dive deeper into the specifics of Bismuth's crystal habit, these crystals can be quite varied in appearance. They can grow up to 12 cm in size, although they are often indistinct and form in parallel groupings. This can result in several intriguing habits, such as:
- Reticulated: Network-like formations that are intricate and interwoven.
- Arborescent: Tree-like structures with branching patterns.
- Foliated: Layered or sheet-like formations.
- Granular: Appearing as small, grain-like aggregates.
These characteristics highlight the versatility and unique charm of Bismuth crystals, making them a fascinating subject for collectors and enthusiasts alike. Whether in their natural state or lab-grown, Bismuth crystals offer a spectrum of forms and hues that captivate the eye.
Naturally occurring Bismuth is not of the purity level required to form the geometrical labyrinths that rainbow Bismuth crystals are prized for. In fact, Bismuth is seldom discovered in nature in its elemental form, making the natural occurrence of pure Bismuth quite rare. This scarcity in nature is one reason why Bismuth Rainbow Crystals are mostly made in labs.
To create these dazzling crystals, it requires heating and concentrating natural Bismuth until it melts. During this process, impurities are meticulously removed. Once the Bismuth is purified, it is allowed to cool, forming the striking geometric shapes and vibrant colors that enthusiasts admire.
By understanding the rarity of elemental Bismuth in nature and the precision needed to produce these crystals, one can truly appreciate the artistry and science behind their creation.
As the temperature of the melted Bismuth lowers, the material solidifies and restructures itself into the intricate, geometric patterns collectors adore. Oxidization causes the rainbow colors to form on the outermost layer of the Bismuth crystal in a tarnish layer, similar to the way that natural Bismuth takes on yellow, pink, and blue tones. While Bismuth itself occurs in nature, it takes human ingenuity and intervention to take the brittle metal and turn it into a dazzling work of art. All of our Bismuth for sale consists of lab grown rainbow Bismuth!
How Bismuth Differs from Minerals like Quartz
Bismuth is unique in the world of elements due to its simple atomic structure. It’s composed entirely of Bismuth (Bi) atoms, distinguishing it from complex minerals.
Take Quartz, for example. Quartz is a compound mineral, consisting of two types of atoms: silicon (Si) and oxygen (O). This combination forms a silicate mineral, which is far more complex in composition compared to pure elemental Bismuth.
Here’s a quick breakdown:
-
Atomic Composition:
- Bismuth: Single element (Bi) only.
- Quartz: Compound of silicon (Si) and oxygen (O).
-
Complexity:
- Bismuth: Simple atomic structure.
- Quartz: Complex structure with multiple types of atoms.
This simplicity in its makeup makes Bismuth stand out from minerals like Quartz and others with varied atomic compositions.
What is the Density of Bismuth?
The density range of Bismuth spans from 9.70 to 9.83 grams per cubic centimeter.
Cleavage and Fracture Properties of Bismuth
When examining the physical characteristics of bismuth, two important properties to consider are its cleavage and fracture.
-
Cleavage: Bismuth displays varied cleavage qualities on different crystallographic planes. It exhibits perfect cleavage on the {0001} plane, indicating that it can split effortlessly and cleanly along this direction. Additionally, bismuth has good cleavage on the {1011} plane, though it is not as easily achieved as on the {0001} plane. The cleavage is less favorable on the {1014} plane, where it is considered poor, making it challenging to achieve a smooth break.
-
Fracture: In contrast to its cleavage properties, the fracture characteristic of bismuth is described as irregular or uneven. This means that when bismuth breaks, it tends not to follow a predictable path and results in a rough and uneven surface.
Overall, understanding these characteristics of bismuth can provide insights into how it behaves under mechanical stress and guide its application in various industries.
Crystallography Properties of Bismuth
Bismuth exhibits unique crystallography properties that distinguish it from other minerals. Here's a breakdown of its key characteristics:
-
Crystal System: Bismuth falls under the trigonal system, specifically exhibiting a hexagonal scalenohedral form.
-
Crystal Habit: The crystals formed by Bismuth can grow up to 12 cm, though they often remain indistinct. They are usually found in parallel groupings and may exhibit hoppered, reticulated, arborescent, foliated, or granular forms.
-
Twinning: Polysynthetic twinning is a common occurrence in Bismuth crystals, contributing to their complex structure.
-
Cleavage: Bismuth displays perfect cleavage in the {0001} plane, good cleavage on the {1011} plane, and poor cleavage on the {1014} plane.
-
Fracture: When fractured, Bismuth exhibits an irregular or uneven break.
-
Tenacity: Bismuth is described as sectile, meaning it can be sliced smoothly with a knife, yet it also possesses a brittle nature.
-
Hardness: On the Mohs hardness scale, Bismuth ranks between 2.0 and 2.5, indicating softness. The Vickers hardness number (VHN100) measures between 16 and 18 kg/mm², detailing its resistance to indentation.
-
Density: The density of Bismuth ranges from 9.70 to 9.83 g/cm³, reflecting its substantial weight relative to size.
These properties make Bismuth an intriguing subject for study in materials science and mineralogy.
To Which Mineral Group Does Bismuth Belong?
Bismuth is a part of the Arsenic Group of minerals, a classification that comprises elements such as Antimony, Arsenic, and of course, Bismuth itself. This group is characterized by its shared properties and chemical similarities, which help in categorizing these elements within the broader mineral classification system.
Understanding Bismuth's Optical Properties
When exploring the optical properties of Bismuth, two key terms come into play: pleochroism and anisotropism.
-
Pleochroism of Bismuth: Bismuth exhibits a very subtle form of pleochroism. This means that there is a slight variation in color when viewed from different angles, but these changes are generally weak and not easily noticeable.
-
Anisotropism of Bismuth: Bismuth showcases distinct anisotropic properties. Under different lighting and angles, the mineral's appearance can shift from a brilliant creamy white to a yellowish hue due to tarnishing. This striking visual variation highlights Bismuth's unique optical characteristics.
Understanding these traits can enhance your appreciation for this fascinating element, whether you're studying it for scientific purposes or simply admiring its natural beauty.
Is Bismuth Radioactive?
Yes, but only very weakly so, and not to any extent that would cause harm. Many everyday objects are more radioactive than Bismuth. In 2003, a groundbreaking discovery revealed that Bismuth, long regarded as the last stable element, is actually radioactive. This finding surprised the scientific community, which had always believed Bismuth to be stable.
Despite this revelation, it has been determined not to pose a health risk. In fact, Bismuth's level of radioactivity is so low that it can't be picked up by most instruments, and until 2003 it was believed to be not radioactive at all.
This subtle radioactivity doesn't affect its practical applications or its safety in everyday use. Therefore, Bismuth continues to be valued in various industries for its unique properties.
Luminescence:
- Bismuth does not exhibit any luminescence. It is devoid of any glow or light emission when exposed to various forms of energy.
Radioactivity:
- While Bismuth is technically radioactive, its radioactivity is extremely weak. This level is insignificant compared to many objects you encounter daily.
- The radioactivity is so minimal that it remained undetectable by most instruments for many years.
- Until 2003, it was assumed to be completely non-radioactive.
The combination of these properties makes Bismuth a safe element to handle, without the risks commonly associated with radioactivity.
What is the half-life of the natural isotope 209Bi?
The natural isotope bismuth-209 boasts an extraordinarily extended half-life. Spanning approximately 19 quintillion years, this impressive duration makes it essentially stable. As a result, even when present in significant amounts, it poses no harm.
As with all crystals and gemstones, you shouldn't rub Bismuth on your eyes, put it up your nose, chew on it, rub sharp edges of Bismuth, or expose it to fire, as these actions could cause injury, but simply holding a piece of Bismuth is not associated with any health risks.
Bismuth Metaphysical Properties and Uses
Rainbow Bismuth resonates through the entire Chakral Column, but is most strongly associated with the Crown Chakra, self empowerment, and transcending limitations. Bismuth is all about reaching out to your Higher Self for support, guidance, and renewed confidence so that you can engage with others from a place of healing. Bismuth is also associated with spiritual awareness and self acceptance, since it offers encouragement for us to find our place in life. The meaning of Bismuth is achieving your highest happiness, focusing on personal growth and wellbeing so that when we're ready, we can resolve conflicts and traumas with grace, clarity, and a centered spirit. Bismuth crystals symbolize blessings, rebirth, and personal evolution, so they make wonderful gifts for loved ones who are embarking on new beginnings to show your unwavering support for their journey.
Characteristics of Bismuth Metal Crystals in Various Weights
Bismuth metal crystals are known for their stunning appearance and high purity. With a purity level of 99.99%, these crystals are perfect for collectors and enthusiasts. One of their most remarkable features is the array of mesmerizing colors on their shiny surfaces.
Weight and Dimension Options
-
20 to 28 Grams: These crystals generally measure about 2 x 4.5 cm. The relatively compact size is perfect for those looking to start or expand their element collection.
-
29 to 44 Grams: Slightly larger, these crystals typically have dimensions around 2.5 x 4.3 cm. They offer a heavier piece for an even more impressive visual display.
-
55 to 103 Grams: The largest available size, these crystals measure approximately 4 x 7 cm. They provide significant visual impact, making them a striking addition to any collection.
Each crystal is a unique, single-piece formation. From small to large, these Bismuth crystals display an incredible range of colors that enhance their natural beauty, making them an appealing choice for both decorative and educational purposes.
What are the Visual Characteristics of Bismuth Crystals?
Bismuth crystals encased in acrylic cubes boast striking visual features that are sure to captivate collectors and enthusiasts alike. Imagine a shiny, iridescent gem that glistens with a spectrum of vibrant colors, reminiscent of a kaleidoscope. These crystals often exhibit a spiraling, staircase-like shape that adds an element of intrigue to their appearance.
Encased within crystal-clear acrylic, these vivid specimens are protected and perfectly preserved for display. The acrylic cube highlights the bismuth's natural luster, allowing light to reflect off the metallic surface and enhance its colorful hues. With purity levels often reaching 99.995%, these samples are not only visually appealing but also boast a high level of authenticity and quality.
In summary, bismuth crystals in acrylic cubes are truly a feast for the eyes, offering collectors a unique and colorful addition to their displays, blending art with science in the most mesmerizing manner.
Discover the Versatile Uses of Bismuth Metal Ingots
Bismuth metal ingots are remarkably pure, often at 99.99%, making them suitable for various practical and scientific applications. Below are some of the fascinating uses of these ingots:
1. Element Collection
- Perfect for Collectors: Due to their high purity, Bismuth metal ingots are an excellent addition to any element collection. Enthusiasts and educators alike appreciate its aesthetic and scientific value.
2. Creation of Bismuth Crystals
- DIY Crystal Formation: Bismuth metal can be easily melted and allowed to cool, resulting in spectacular, iridescent crystals. This process can be a captivating experiment for hobbyists and students exploring crystallization and metallurgy.
3. Laboratory and Industrial Applications
- Scientific Research: In laboratories, Bismuth's low toxicity and interesting properties make it useful for various experiments and educational purposes.
- Manufacturing and Industry: Bismuth is often used in creating alloys, particularly where lead replacement is desired due to its non-toxic nature. It's employed in the production of low-melting-point metals used in fire-detection and extinguishing systems.
4. Art and Design
- Innovative Art Projects: Artists are drawn to Bismuth for its unique appearance and ease of shape transformation. The metal's ability to create striking and colorful designs drives its popularity in jewelry and decorative art.
With its blend of aesthetic appeal, scientific intrigue, and practical applications, Bismuth metal ingots are truly versatile, serving a broad spectrum of purposes from education to industry.
What is a Notable Use of Bismuth Metal
A significant application of bismuth metal is its use in alloys with low melting points. These alloys are particularly useful in safety devices, such as fire sprinkler systems, where precise melting at lower temperatures is crucial. Additionally, bismuth's unique properties make it a popular choice in the production of fusible alloys used in various manufacturing industries. Its ability to replace lead in many applications adds to its appeal, especially in environmentally conscious settings.
Unlocking the Potential of Bismuth Metal in Educational Settings
Bismuth metal, renowned for its unique properties, offers exciting opportunities in educational environments. This element serves as a tangible educational tool across various academic disciplines, engaging students and enhancing learning experiences.
Hands-On Learning in Science Classrooms
Bismuth's distinctive characteristics make it ideal for demonstrating essential scientific concepts:
- Density and Volume Exploration: A Bismuth cube, precisely measuring 10x10x10mm and weighing approximately 9.78 grams, can introduce students to density calculations and the principles of mass and volume.
- Elemental Studies: As a pure metal, Bismuth becomes a valuable asset for lessons on the periodic table, showcasing metal properties such as crystalline structure and heat conductivity.
Valuable Resource for Schools and Universities
Educational institutions can integrate Bismuth into various aspects of their curriculum:
- Chemistry Experiments: Use Bismuth to explore reactions and compounds, helping students observe chemical changes and compound formation.
- Advanced Physics Demonstrations: Highlight phenomena like thermoelectric effects, allowing students to see practical applications of physics theories.
Inspiring Collectors and Future Scientists
For young enthusiasts and aspiring scientists, Bismuth fosters curiosity and inspires future exploration:
- Element Collections: As part of a wider collection of elements, Bismuth adds a tangible, real-world aspect to theoretical studies, encouraging deeper interaction with chemistry.
- Student Projects and Competitions: Students can use Bismuth in various science fairs or competitions, applying theoretical knowledge to real-world scenarios.
Bring Bismuth into your classroom to stimulate interest, enhance curriculum, and foster a deeper understanding of scientific principles.
What is the Chemical Symbol and Atomic Number of Bismuth?
Bismuth is represented by the chemical symbol "Bi" on the periodic table and bears the atomic number 83.
Refractive Index and Birefringence Properties of Bismuth
Bismuth exhibits a unique set of optical properties that make it stand out, particularly in its use for optical applications.
Refractive Index
The refractive index of Bismuth changes with different wavelengths of light, demonstrating its versatility:
- At 400 nm: The index ranges from 47.0 to 58.2
- At 420 nm: It shifts to between 49.3 and 58.8
- At 440 nm: The range lies between 51.4 and 59.7
- At 460 nm: It moves to 52.9–60.9
- At 480 nm: The span is from 54.4 to 62.4
- At 500 nm: The index is between 56.2 and 63.9
- At 520 nm: It ranges from 57.8 to 65.3
- At 540 nm: The scale is 59.3 to 66.6
- At 560 nm: The index is 60.4 to 67.8
- At 580 nm: It is between 61.4 and 69.0
- At 600 nm: The index ranges from 62.4 to 69.9
- At 620 nm: It lies between 63.1 and 70.7
- At 640 nm: The range is 63.6 to 71.5
- At 660 nm: It shifts to 63.9–72.2
- At 680 nm: The range is 64.0 to 72.8
- At 700 nm: It tops out at 64.1 to 73.2
These variations indicate Bismuth's potential in high-precision optics where specific wavelengths are crucial.
Birefringence
In terms of birefringence, Bismuth is characterized as opaque with a birefringence value of 0.00. This means that when Bismuth is examined under polarized light, it doesn't show double refraction, which might be beneficial or limiting depending on the intended application.
Understanding these optical properties can significantly impact the decision-making process for industries involved in optical components and technologies.
What Makes Bismuth Unique in Terms of Magnetism?
Bismuth stands out in the realm of minerals due to its exceptional magnetic properties. Unlike most elements, bismuth is diamagnetic, which means it creates an internal magnetic field that opposes external magnetic forces. This characteristic results in bismuth being repelled by magnetic fields rather than attracted.
Key Characteristics of Bismuth:
-
Diamagnetism: Bismuth is one of the most diamagnetic elements known. This property causes it to repel magnetic fields, a behavior not commonly observed in metals.
-
Thermal Conductivity: Among metals, bismuth has an exceptionally low thermal conductivity, which adds to its unique profile.
In contrast, minerals like Xenotime exhibit paramagnetism, where they are attracted to external magnetic fields and align with these forces, a completely different behavior from that of bismith. This comparison highlights bismith's distinctive place in the world of magnetism.
What Is the Chemical Formula and Molecular Weight of Bismuth?
The chemical formula for Bismuth is Bi, which indicates it as a native element. Its molecular weight is approximately 208.98 grams per mole.
Where Are Some of the Localities Where Bismuth is Found?
Bismuth can be found in various locations around the globe. In Germany, you can find it in Altenberg, Schneeberg, and Annaberg located in Saxony. The Czech Republic is home to bismuth at Jáchymov (also known as Joachimsthal). Spain offers sites near Villanueva de Córdoba in the Córdoba Province. England contributes its share from the Dolcoath and other mines in Cornwall.
In South America, Bolivia has economically significant deposits at locations such as Uncia, Chorolque, Llallagua, and Tazna in the Potosí region, with a notable 11 kg nugget discovered in Velasquez, La Paz. Australia also boasts bismuth at the Mt. Arthur mine in Queensland and from Kingsgate in New South Wales. Japan presents large crystals in Natsukidani, located in the Oita Prefecture.
Lastly, Canada has its share of bismuth too, particularly from sources in Cobalt, Ontario. These diverse sites underscore bismuth’s widespread presence across continents.
What is a Hopper Crystal and How Does it Form?
A hopper crystal is a fascinating type of crystal formation characterized by its distinctive shape. Unlike typical crystals with smooth surfaces, hopper crystals have hollow, stepped faces that resemble a staircase. These edges extend outward while the internal structure remains incomplete, giving them their unique appearance.
Hopper crystals form due to an imbalance in growth rates between the edges and the faces of the crystal. During the crystallization process, the edges grow more rapidly than the central areas. As a result, the outer edges develop fully, while the interior lags behind, creating the signature stepped, hollow look. This phenomenon occurs in various minerals, including salt, quartz, and more.
Understanding the Difference Between Diamagnetism and Paramagnetism
Diamagnetism and paramagnetism are two interesting phenomena that describe how materials respond to magnetic fields, yet they differ significantly in their behavior.
Diamagnetism is a type of magnetism found in certain materials that causes them to be repelled by an external magnetic field. This effect occurs because diamagnetic materials create an internal magnetic field that opposes the direction of the applied magnetic field. Essentially, when a diamagnetic substance, such as bismuth or copper, is placed in a magnetic field, it generates a weak opposing field, leading to a repulsive force.
On the other hand, Paramagnetism refers to a form of magnetism where materials are attracted to an external magnetic field. Unlike diamagnetic substances, paramagnetic materials produce internal magnetic fields that align with the direction of the applied field, which enhances the attraction. Examples of paramagnetic materials include aluminum and platinum.
Here's a quick breakdown of the differences:
-
Nature of Interaction:
- Diamagnetism: Repelled by magnetic fields.
- Paramagnetism: Attracted to magnetic fields.
-
Internal Field Orientation:
- Diamagnetism: Opposes the external field.
- Paramagnetism: Aligns with the external field.
Understanding these nuances helps in identifying the behavior of various minerals and metals when exposed to magnetic environments.
What Are Native Element Minerals?
Native element minerals are minerals composed of a single element in an uncombined and pure form, characterized by a uniform mineral structure. These minerals occur naturally in the Earth's crust and are distinct because they do not bond with other elements to form compounds.
Characteristics of Native Element Minerals:
- Purity: These minerals consist of a single type of atom. For instance, gold (Au) and silver (Ag) are examples where the entire mineral is composed solely of one element.
- Natural Occurrence: They are formed under specific geological conditions and are not artificially created or chemically altered.
- Distinct Structure: Each native element mineral possesses a unique crystal structure that defines its physical properties, like hardness and density.
Common Examples:
- Gold (Au): Known for its luster and malleability, often found in nugget form or embedded within rocks.
- Copper (Cu): Identified for its reddish hue and conductivity, frequently used in electrical applications.
- Sulfur (S): Typically yellow and utilized in industrial processes, found near volcanic regions.
In summary, native element minerals are fascinating due to their simplicity and the unique conditions under which they form, making them essential study subjects in geology and valuable resources in various industries.